In a recent development that has captured the attention of healthcare professionals and patients alike, the U.S. Food and Drug Administration (FDA) has issued a warning regarding counterfeit Ozempic, a medication widely prescribed for the treatment of type 2 diabetes and, in some cases, used off-label for weight loss.
The Houston pharmaceutical injury attorneys at Reich & Binstock understand the dangers of counterfeit medications and their impact on those who unknowingly take them. That’s why our law firm is at the forefront of addressing the legal implications of this warning, advocating for those who may have experienced adverse effects from fake Ozempic.
In this blog, our attorneys shed light on the nature of the FDA’s concerns, the potential health risks associated with counterfeit Ozempic, and the legal avenues available for affected individuals seeking justice and compensation.
If you’ve suffered the consequences of counterfeit Ozempic or other weight loss drugs, contact Reich & Binstock at (713) 622-7271 to meet with one of our experienced Ozempic lawyers today.
What Is Ozempic?
Ozempic is a prescription medication that has received significant attention in the medical community for its role in managing type 2 diabetes. It contains the active ingredient semaglutide and functions as a glucagon-like peptide-1 (GLP-1) receptor agonist, a class of drugs that mimic the action of the GLP-1 hormone, which plays a crucial role in regulating blood sugar levels.
By stimulating insulin production in response to high blood glucose levels and reducing the amount of sugar produced by the liver, Ozempic effectively helps to control blood sugar levels in adults with type 2 diabetes. Additionally, this diabetes drug has been associated with weight loss, leading some healthcare providers to prescribe it off-label for this purpose, further increasing its popularity and usage.
What Are the Dangers of Ozempic?

While Ozempic has proven effective for managing type 2 diabetes and has gained popularity for its weight loss benefits, it is not without risks and potential side effects. But what are the bad side effects of Ozempic? Some users have reported mild to severe reactions, ranging from nausea, vomiting, diarrhea, stomach pain, and constipation, which are some of the known common adverse reactions.
However, more serious reactions have been reported, like pancreatitis, kidney problems, and an increased risk of diabetic retinopathy complications in those with a history of diabetic eye disease.
Gastroparesis
Gastroparesis is a long-term medical condition marked by a slowdown in the stomach’s ability to empty food into the small intestine without any physical blockage. This condition interferes with the stomach muscles’ usual rhythmic contractions, causing symptoms including persistent nausea, vomiting, abdominal pain, feelings of fullness soon after starting a meal, and bloating. In more extreme cases, it can cause significant nutritional deficiencies and a reduction in body weight as it struggles to absorb and process nutrients efficiently.
This medical condition has occurred in many individuals who are taking Ozempic, as the medication affects gut mobility and glucose regulation. While Ozempic effectively manages blood sugar levels in type 2 diabetes, its mechanism of action, which involves slowing gastric emptying as part of its glucose-lowering effect, could lead to patients developing gastroparesis.
The FDA Warns Consumers Not to Use Counterfeit Ozempic
The FDA has cautioned consumers about the risks of using counterfeit versions of Ozempic. These fake products, which may be circulating in the market without proper authorization, pose significant health risks due to the potential lack of active ingredients, incorrect dosages, or the presence of harmful substances.
The FDA’s alert stresses the importance of obtaining Ozempic through legitimate healthcare channels and pharmacies to ensure the safety and effectiveness of the medication. This warning highlights the broader issue of counterfeit medications, which not only jeopardize patient health but also undermine trust in medical treatments and the pharmaceutical supply chain.
Users Should Report Adverse Events to the FDA
The five adverse events, or side effects, that are related to fake Ozempic include diarrhea, nausea, and vomiting. However, these are the same adverse events that can occur with authentic Ozempic. It’s important to monitor these signs and report them to the FDA’s MedWatch program, which is a platform for healthcare professionals and consumers to document serious problems they suspect may be associated with the medications and medical devices they prescribe, dispense, or use.
As of December 2023, the FDA was still investigating counterfeit Ozempic and strongly urged retail pharmacies and patients to check the products they’ve received. The agency identified the lot number (NAR0074) and the serial number (430834149057) of the affected products but warned they could still be available. To increase consumer awareness and safety, the FDA has provided side-by-side images of an authentic Ozempic needle and a counterfeit needle.
Reporting incidents and suspected counterfeit products is essential for continuously monitoring drug safety and usefulness. These reports allow the FDA to assess the impact of counterfeit medications, identify potential dangers not found during standard regulatory processes, and take appropriate actions to protect public health.
What Is the New Black Box Warning on Ozempic?
A black box warning is the strictest caution issued by the FDA and is placed on prescription drugs that may cause serious or life-threatening risks. The black box warning that the FDA has added to Ozempic and other semaglutide products cautions users of the increased risk of developing thyroid tumors and thyroid cancer. This warning stems from rodent studies that resulted in the animals developing thyroid C-cell tumors. However, the risks for human patients developing thyroid C-cell tumors have not yet been determined.
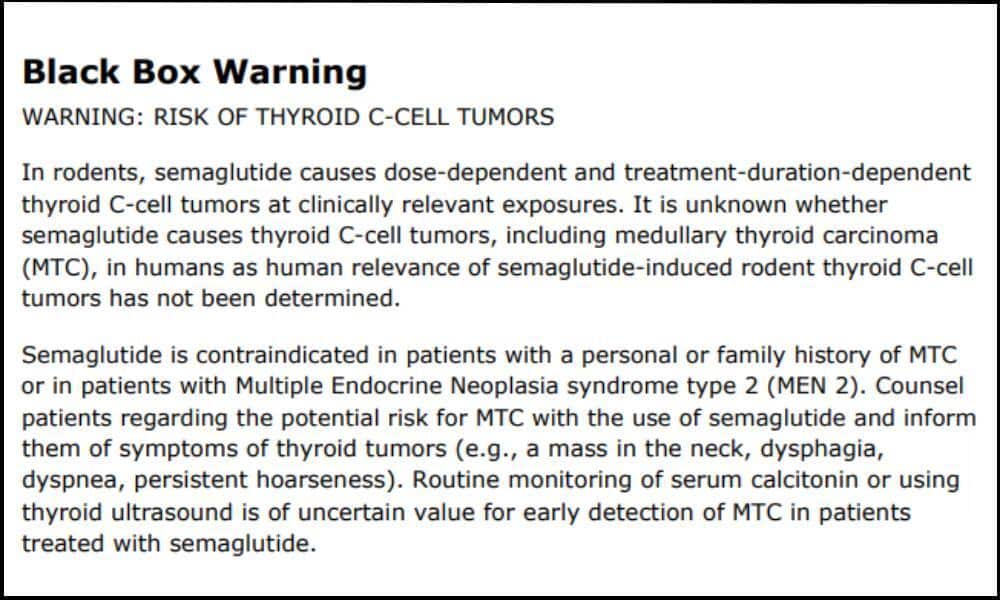
Photo Credit: Texas Department of Health and Human Services
Contact an Ozempic Lawsuit Attorney with Reich & Binstock
If you’ve experienced adverse effects from using Ozempic, whether due to the medication itself or counterfeit versions, it’s crucial to understand your legal rights and options. The experienced Ozempic lawsuit attorneys at Reich & Binstock are dedicated to supporting individuals affected by pharmaceutical injuries, offering expert legal counsel and representation.
Contact Reich & Binstock today at (713) 622-7271 to schedule a consultation and take the first step toward holding responsible parties accountable.














